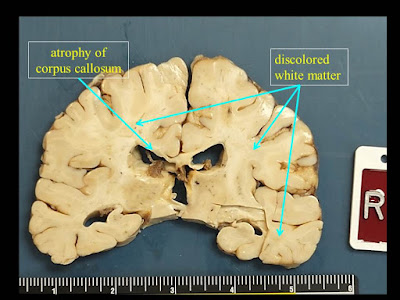
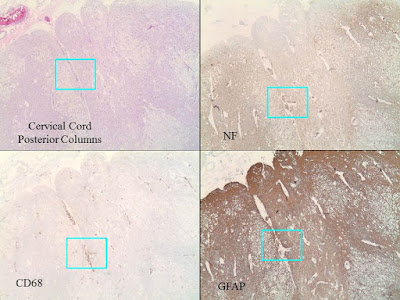
|
| Karra Jones, MD, PhD |
From time to time on Neuropathology Blog, we profile a prominent neuropathologist. In the past, we've featured the likes of
Craig Horbinski,
Roger McLendon, and
Jan Leestma. Today, we feature a rising star in the field:
Karra A. Jones, MD, PhD. Having just moved to the
University of Iowa from
UCSD, Dr. Jones is poised to do great work at her new institution. Here's a short bio followed by a Q&A with the inimitable Dr. Jones:
Karra Jones grew up in Kansas City where she completed her M.D. and Ph.D. at the University of Kansas School of Medicine. Karra’s graduate work focused on the evaluation of muscle spindle innervation by large peripheral nerve fibers and proprioceptive abnormalities in diabetes. During her time at KUMC, Karra was inspired by the strong history of neuropathology in Kansas City started by the dearly missed John Kepes and continued by her amazing mentor Kathy Newell. Karra traveled to the West Coast in 2010 to train in combined anatomic pathology/neuropathology under Lawrence Hansen, Scott VandenBerg, Subhojit Roy, and Henry Powell at the University of California, San Diego. There she focused on brain tumor research with Scott VandenBerg and Steve Gonias and developed a clinical interest in neuromuscular pathology. She was fortunate to obtain additional training in muscle pathology at UCSD with Diane Shelton in The Comparative Neuromuscular Laboratory. Karra joined the staff at UCSD in 2014 where she headed the neuromuscular service, participated in the general neurosurgical service, collaborated with molecular pathology on brain tumor molecular testing protocols/testing, supervised a biomarker laboratory, and was a co-director of the tissue biorepository. Karra very recently returned to the Midwest to join the highly talented neuropathology group at the University of Iowa where she is very excited to be practicing alongside Steve Moore, Leslie Bruch, Pat Kirby, and Gary Baumbach.
1. Why did you decide to become a neuropathologist?
I became interested in the neurosciences after spending a year as a research assistant at Emory University in the Department of Neurology prior to medical school. Then, during graduate school at KUMC, my interest in tissue morphology was peaked after spending hours each day under a confocal microscope staring at muscle spindle innervation (what a gorgeous thing!) While at KUMC, I was extremely lucky to have Kathy Newell take me on as a mentee, and after that I was hooked. Almost everyone in my family is an artist, and I often felt like the outsider in that regard. But I realized with pathology, and in particular the beauty of neuropathology, I was a different kind of artist in my own right. Examining, classifying, and photographing brain tumors, neuromuscular diseases, and neurodegenerative diseases seemed like the most fun I could ever have at work. And I continue to have fun every day as a neuropathologist.
2. Name a couple of important professional mentors. Why were they important to you?
I already mentioned Kathy Newell – Kathy has been an amazing mentor throughout my training and early career even though I haven’t worked with her directly since medical school. She first inspired me to pursue neuropathology with her amazing eye, calm demeanor, and kind heart. She also taught me about the importance of a “Neuropathology Family” introducing me to John Kepes and encouraging me to work with B.K. DeMasters during my last year of medical school, which helped solidify my decision to pursue combined AP/NP training. Another very important mentor is Lawrence Hansen (although he would argue that mentor means “cross-dresser” as the word is derived from Homer’s Odyssey in which Athena assumes the form of Mentor.) Larry is one of the most talented teachers and morphologists I have ever had the opportunity to work with. His “Hansen-isms” are embedded in my brain for life and as a neuropathologist and educator I will forever pass them on to my fellows, residents, students and mentees. Not only is Larry an amazing teacher and mentor, but also he is a very good friend and human being. I was also extremely lucky to be mentored by Scott VandenBerg on brain tumor diagnosis, molecular testing, and basic science research. Without Scott’s influence, I wouldn’t be where I am today.
3. What advice would you give to a pathology resident interested in doing a neuropathology fellowship?
Do it! Neuropathology is clearly the best of all pathology specialties. But in all seriousness, Neuropathology training will give you a highly desirable skill set that will prepare you for a large variety of career paths. There are many ways to “differentiate” as a neuropathologist – academic, private practice, research, clinical, tumors, neuromuscular, neurodegenerative, etc. So, prior to your NP training, try to think about what you would like to do as a career after it’s all said and done, but remember to always be flexible and allow yourself to change your mind (it happens in medicine quite often). Neuropathology can also be a good specialty to combine with others such as pediatric pathology and forensic pathology, making you a highly desirable job candidate for varied positions. Don’t be intimidated by the 2 year commitment of the NP fellowship. One extra year in training is nothing in the grand scheme of life and only prepares you even better for the day you click “finalize” on your first case (or it gives you more time to work toward getting grant funding before the clock starts ticking). Right now, there are many job openings in neuropathology – we need bright, motivated, and enthusiastic trainees to become the next generation of neuropathologists.
4. What city would you like a future American Association of Neuropathologists meeting to be held and why?
I would love for the meeting to be held in San Francisco again. I love visiting the city and always look for excuses to return.